NashBio has worked with a wide-ranging selection of clients that leverage our expansive dataset and expertise to develop innovative solutions for their research. Here are just a few examples demonstrating how our work produced key insights and breakthroughs. Whether it’s a project to identify novel drug targets using or a project to define patient outcomes, we offer intelligent approaches, data, and tools that enhance discoveries.
Case Studies
Case Studies
NashBio has worked with a wide-ranging selection of clients that leverage our expansive dataset and expertise to develop innovative solutions for their research. Here are just a few examples demonstrating how our work produced key insights and breakthroughs. Whether it’s a project to identify novel drug targets using or a project to define patient outcomes, we offer intelligent approaches, data, and tools that enhance discoveries.
Disease Risk Modeling
Acute pancreatitis has become increasingly prevalent across the world, affecting nearly 85,000 individuals annually. Patients with acute pancreatitis experience significant rates of mortality in the emergency department (ED) and currently, there are no known drug treatments for the disease. Even though several long-term pancreatitis scoring systems exist (Ranson’s criteria, APACHE-II score, and the BISAP index), there is a paucity of early prognostic scores that apply to the ED triage.
Send download link to:
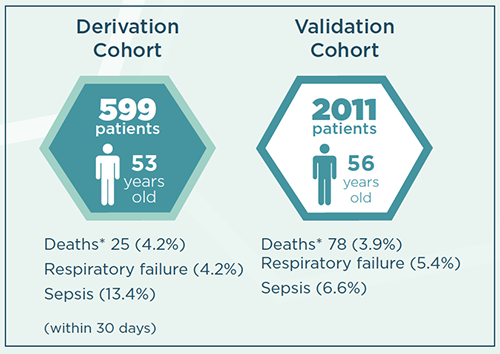
Assessing pain-associated SNPs through the linkage of genetic and clinical information
Hereditary neuropathies are a group of peripheral nervous system disorders that are characterized by numbness, tingling, and pain in the feet and/ or hands1. The four main subcategories of hereditary neuropathy include motor, sensory, motor and sensory, or sensory and autonomic. As the name suggests, hereditary neuropathy has a genetic component; however, the degree of severity varies from person to person with some being more severely affected than others.
Send download link to:
Synthetic Control Arm to Boost Clinical Development
Nashville Biosciences recently worked with a clinical stage biotech to develop a synthetic control arm for its active single-arm clinical trial in a rare but deadly cancer. The goal was to identify a virtual cohort of subjects that matched the selection criteria for the company’s actual clinical trial. We then studied this cohort to measure how they responded to current standard of care treatments and the outcomes they achieved. Download the case study to read more about our approach and how it can impact clinical development.
Send download link to:

Measuring the Impact of New Drug Treatments
Nashville Biosciences recently worked with our partners Vital Transformation to understand the impact of a newly-launched treatment for a type of leukemia. Using our extensive real-world data, we identified subject cohorts that received this new therapy and that received the historical standard of care. Vital Transformation then compared how subjects in each cohort responded to treatment and what other medical care they required. Download the case study to read more about our approach and learn about our results.
Send download link to:
Improving Early-Stage Drug Discovery Process
Nashville Biosciences recently worked with Pfizer’s Target Sciences and Technologies group to explore genetic targets for treating a serious metabolic disease with greatly increasing prevalence. The goal was to leverage BioVU® to rapidly select disease and disease-protected subject cohorts with multiple long-term clinical risk factors, and then perform DNA sequencing to identify new variants or mutations to inform further drug R&D. Download the case study learn more about this unique study design and how we used BioVU® to improve the early-stage drug discovery process.
Send download link to:
Investigating genetic variants to guide neuropathic pain perception R&D
Nashville Biosciences recently worked with a top-twenty pharmaceutical company to prioritize 20 early stage targets. The goal was to identify potential new therapeutic indications that fit with the company’s disease area priorities by using phenome wide association studies (pheWAS). A pheWAS-driven portfolio prioritization is only possible with a resource like BioVU® that combines a large population of prospectively genotyped subjects with a rich set of EMR-derived clinical phenotypes. Download the case study to find out how we accomplished this to help build a better drug portfolio.
Send download link to:
Early Stage Drug Target Prioritization
Nashville Biosciences recently worked with a top-twenty pharmaceutical company to prioritize 20 early stage targets for new drug discovery or to repurpose existing drugs already in development. The goal was to identify potential new therapeutic indications that fit with the company’s disease area priorities by using phenome wide association studies (pheWAS). A pheWAS-driven portfolio prioritization is only possible with a resource like BioVU® that combines a large population of prospectively genotyped subjects with a rich set of EMR-derived clinical phenotypes. Download the case study to find out how we accomplished this to help build a better drug portfolio.
Send download link to:
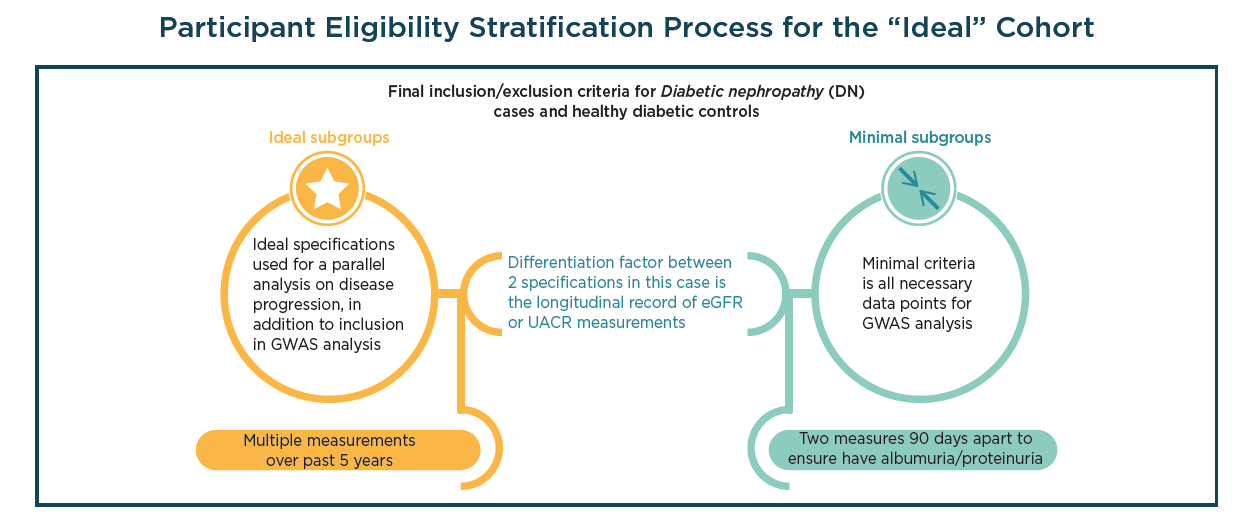
Utilizing GWAS to investigate chronic kidney disease
Diabetic nephropathy (DN), a chronic condition that occurs in patients with diabetes mellitus, is a major factor chronic kidney disease and end-stage renal failure. Given the increasing prevalence of type 2 diabetes (T2D) coupled with the fact that DN occurs in 25% of T2D adults, the condition has become the biggest cause of end-stage renal disease and requires additional investigation in order to develop novel therapeutics designed to combat the disease.
Send download link to:
Pharmacovigilance
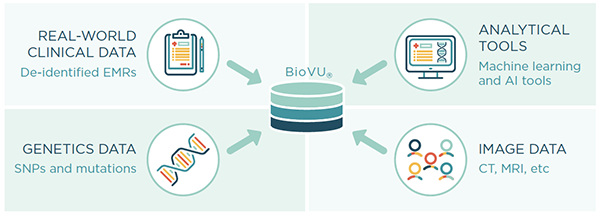
Aggregating millions of adverse event reports to make meaningful conclusions on a drug, disease, or treatment is challenging, and individual case safety reports do not give the full patient medical history. Furthermore, it can be difficult to determine whether an adverse event is a singular occurrence or is likely to affect a wider population.
Nashville Biosciences is well positioned to aggregate real-world data on patient cohorts with longitudinal medical records. Leveraging our unique algorithms and BioVU®, our database of genetically-linked, de-identified electronic health records with over 3 million individual’s EMRs spanning an average length of 12 years, we can rapidly generate valuable insights to address pharmacovigilance needs.
Send download link to:

