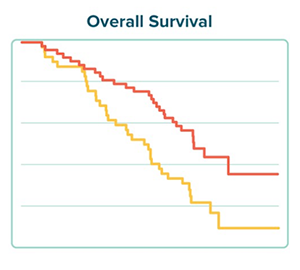
Nashville Biosciences recently worked with a clinical stage biotech to develop a synthetic control arm for its active single-arm clinical trial in a rare but deadly cancer. The goal was to identify a virtual cohort of subjects that matched the selection criteria for the company’s actual clinical trial. We then studied this cohort to measure how they responded to current standard of care treatments and the outcomes they achieved. Download the case study to read more about our approach and how it can impact clinical development.
Synthetic Control Arm to Boost Clinical Development
Send download link to:

Measuring the Impact of New Drug Treatments
Nashville Biosciences recently worked with our partners Vital Transformation to understand the impact of a newly-launched treatment for a type of leukemia. Using our extensive real-world data, we identified subject cohorts that received this new therapy and that received the historical standard of care. Vital Transformation then compared how subjects in each cohort responded to treatment and what other medical care they required. Download the case study to read more about our approach and learn about our results.
Send download link to: