Aggregating millions of adverse event reports to make meaningful conclusions on a drug, disease, or treatment is challenging, and individual case safety reports do not give the full patient medical history. Furthermore, it can be difficult to determine whether an adverse event is a singular occurrence or is likely to affect a wider population.
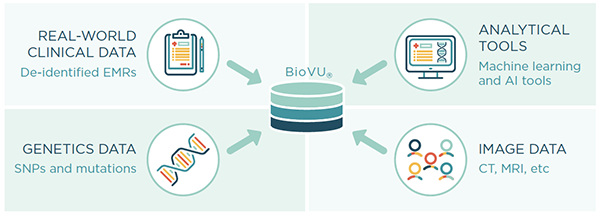
Nashville Biosciences is well positioned to aggregate real-world data on patient cohorts with longitudinal medical records. Leveraging our unique algorithms and BioVU®, our database of genetically-linked, de-identified electronic health records with over 3 million individual’s EMRs spanning an average length of 12 years, we can rapidly generate valuable insights to address pharmacovigilance needs.